With all our might, the process of mass production of the vaccine continues
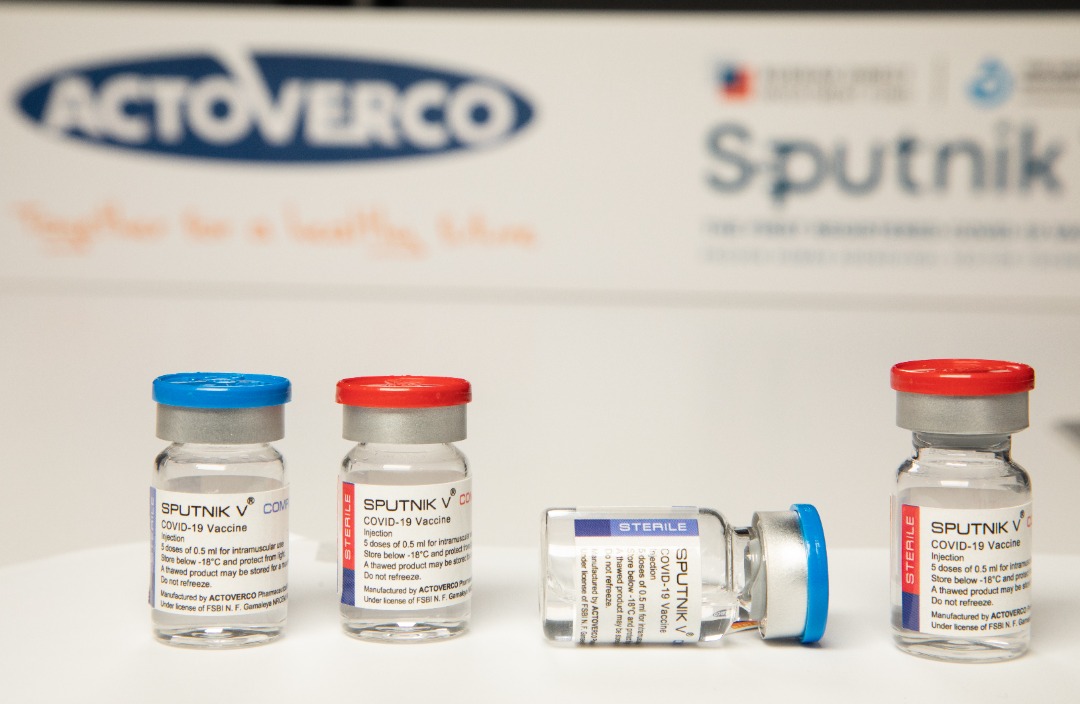
In the name of God, the merciful, the compassionate With the God’s approval and the efforts of the elite young people of our beloved country, the process of producing Sputnik vaccine in the Actoverco production lines was successfully completed. All equipment and production machinery of this product are available in the production lines of Actoverco Biotech and also have been evaluated and approved by the Russians. Accordingly, all control and quality tests regarding the originality and efficiency of the product have been terminated in the laboratories of this corporation with complete success. Based on international protocols and signed contracts between the Russian Direct Investment Fund (RDIF) and all worldwide production contracting parties, the first batches must be sent to the Gamaleya Institute of Russia for final approval so that they can issue the release license of the product. Actoverco pharmaceutical group, besides the denial of all rumors, informs the honorable people of Iran that unfortunately, in the meeting that was held by the current government with the elected President, the representatives of Actoverco pharmaceutical company were not invited; though, these negative and biased measures and competitions, as well as preventing the provision of required financial resources, have not make the Actoverco insecure in the path of providing the vaccine to the dear people, and this group, with hope in God Almighty and relying on its native knowledge, continues the track of evolution and service powerfully. According to the time table, after receiving the above-mentioned approval, the company will provide900000 doses of Sputnik V vaccine or3.5 million doses of Sputnik Light vaccine per month to the Ministry of Health so they can offer it publicly according to the distribution protocol.
In the name of God, the merciful, the compassionate
With the God’s approval and the efforts of the elite young people of our beloved country, the process of producing Sputnik vaccine in the Actoverco production lines was successfully completed. All equipment and production machinery of this product are available in the production lines of Actoverco Biotech and also have been evaluated and approved by the Russians. Accordingly, all control and quality tests regarding the originality and efficiency of the product have been terminated in the laboratories of this corporation with complete success. Based on international protocols and signed contracts between the Russian Direct Investment Fund (RDIF) and all worldwide production contracting parties, the first batches must be sent to the Gamaleya Institute of Russia for final approval so that they can issue the release license of the product.
Actoverco pharmaceutical group, besides the denial of all rumors, informs the honorable people of Iran that unfortunately, in the meeting that was held by the current government with the elected President, the representatives of Actoverco pharmaceutical company were not invited; though, these negative and biased measures and competitions, as well as preventing the provision of required financial resources, have not make the Actoverco insecure in the path of providing the vaccine to the dear people, and this group, with hope in God Almighty and relying on its native knowledge, continues the track of evolution and service powerfully. According to the time table, after receiving the above-mentioned approval, the company will provide900000 doses of Sputnik V vaccine or3.5 million doses of Sputnik Light vaccine per month to the Ministry of Health so they can offer it publicly according to the distribution protocol.